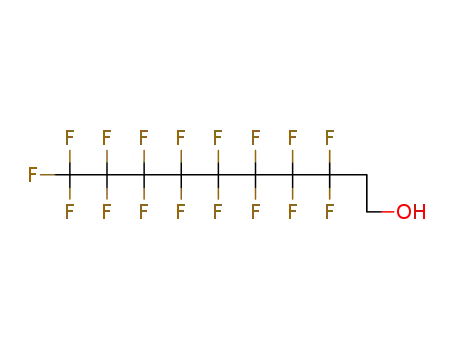
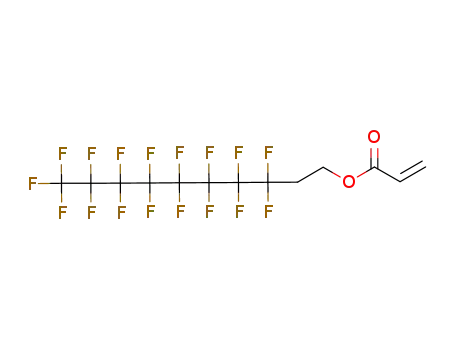
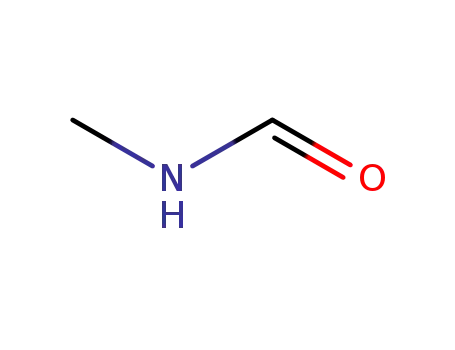
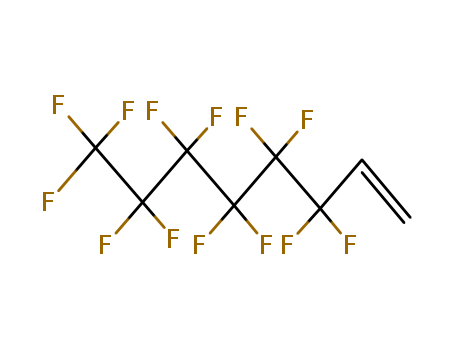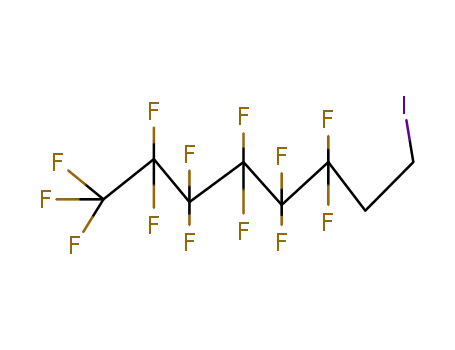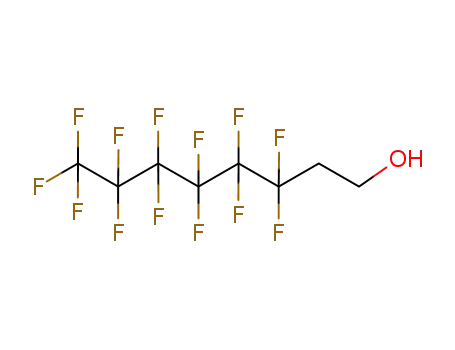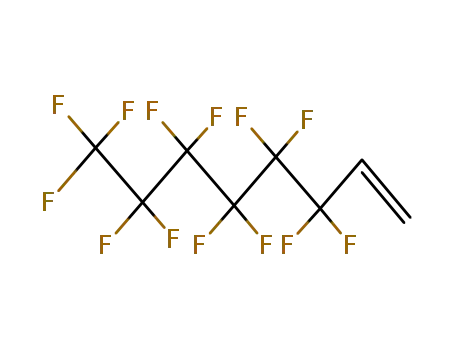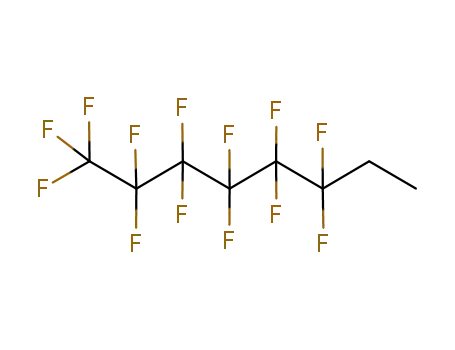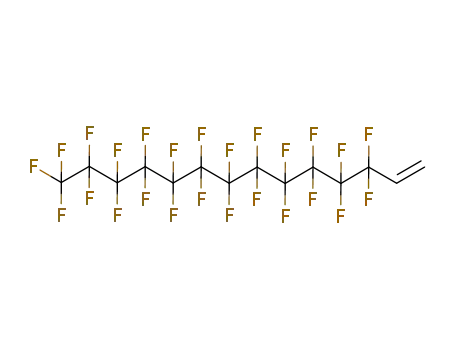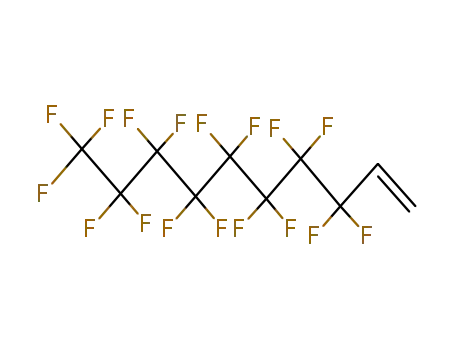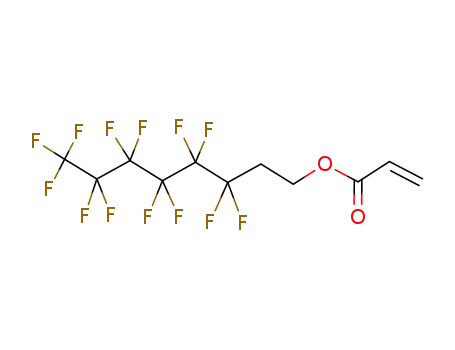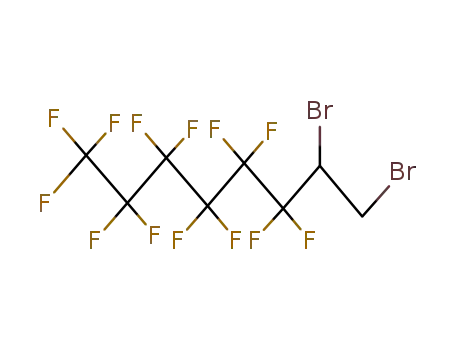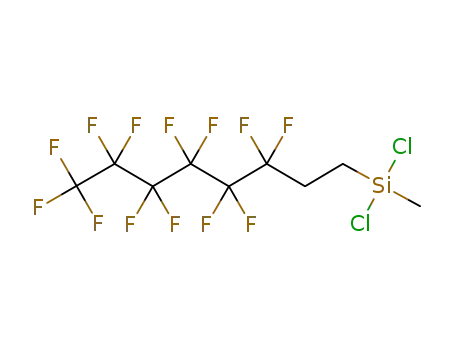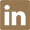Buy Reliable Quality (Perfluorohexyl)ethylene 25291-17-2 Offer with Efficient Delivery
- Molecular Formula:C8H3 F13
- Molecular Weight:346.091
- Appearance/Colour:Colorless liquid.
- Vapor Pressure:43.8mmHg at 25°C
- Refractive Index:n20/D 1.295(lit.)
- Boiling Point:99.5 °C at 760 mmHg
- Flash Point:20 ºC
- PSA:0.00000
- Density:1.52
- LogP:4.91120
(Perfluorohexyl)ethylene(Cas 25291-17-2) Usage
|
Chemical Properties
|
clear colorless liquid
|
|
Flammability and Explosibility
|
Notclassified
|
InChI:InChI=1/C8H3F13/c1-2-3(9,10)4(11,12)5(13,14)6(15,16)7(17,18)8(19,20)21/h2H,1H2
25291-17-2 Relevant articles
Parahydrogen-induced polarization transfer to 19F in perfluorocarbons for 19F NMR spectroscopy and MRI
Plaumann, Markus,Bommerich, Ute,Trantzschel, Thomas,Lego, Denise,Dillenberger, Sonja,Sauer, Grit,Bargon, Joachim,Buntkowsky, Gerd,Bernarding, Johannes
, p. 6334 - 6339 (2013)
Fluorinated substances are important in ...
RADICAL SYNTHESIS OF PERFLUORO-n-HEXYL-2-ETHANOL
Signe, E.,Blancou, H.,Benefice-Malouet, S.,Itier, J.
, p. 289 (1991)
-
SYNTHESE DE PERFLUORO-nHEXYL-2 ETHANOL C6F13C2H4OH PAR REDUCTION ELECTROCHIMIQUE SUR FIBRES DE CARBONE DE PERFLUORO-nHEXYL-2 IODO-1 ETHANE C6H13C2H4I DANS LE SOLVANT N,N DIMETHYLFORMAMIDE
Benefice-Malouet, S.,Blancou, H.,Calas, P.,Commeyras, A.
, p. 245 - 260 (1988)
Electrochemical reduction of 2-perfluoro...
Fluorinated imidazolium salts having liquid crystal characteristics
Zama, Isabella,Gorni, Giacomo,Borzatta, Valerio,Cassani, Maria Cristina,Crupi, Cristina,Di Marco, Gaetano
, p. 749 - 753 (2016)
A family of fluorinated imidazolium salt...
2-(Perfluoroalkyl)ethanols by thermal alkylation of ambidentate lactams with 2-(perfluoroalkyl)-1-iodoalkanes, in the presence of added water. A change in mechanism and stoichiometry of the reaction. Isolation of a water adduct of the lactim ether intermediate
Brace
, p. 6504 - 6516 (1996)
Thermal alkylation of amides by an alkyl...
Quaternary ammonium ionic liquids containing fluorous ponytails: Competitive alkylation and elimination reactions of I(CH2) nRf (n = 2, 3) with tertiary amines
Alhanash, Hana B.,Brisdon, Alan K.
, p. 152 - 157 (2013)
The formation of quaternary ammonium iod...
Study of the alkylation of chlorosilanes. Part III. Synthesis and reactivity of new fluorinated organolithium reagents
Boutevin, B.,Guida-Pietrasanta, F.,Ratsimihety, A.,Caporiccio, G.
, p. 53 - 58 (1995)
The effect of the structure of the fluor...
Method for synthesizing perfluoroalkyl ethylene
-
Paragraph 0028-0030, (2018/02/04)
The invention provides a method for synt...
25291-17-2 Process route
-
- 2043-57-4,71215-70-8
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane
-
- 647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol
-
- 25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene
-
- 80793-17-5
(perfluoro-n-hexyl)ethane
Conditions
| Conditions |
Yield |
|
With water; lithium chloride; In N,N-dimethyl-formamide; Product distribution; effect of quantity of water and current on electroreduction;
|
80 % Spectr.
5 % Spectr.
15 % Spectr. |
-
- 116-14-3,82785-14-6
polytetrafluoroethylene
-
- 354-64-3
Pentafluoroethyl iodide
-
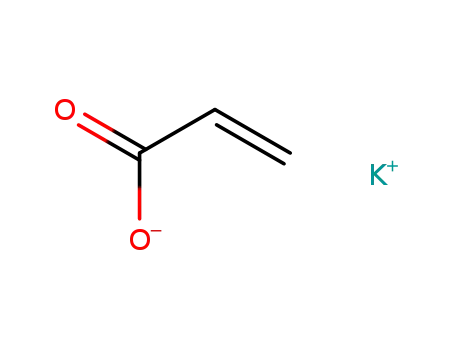
- 10192-85-5
potassium acrylate
-
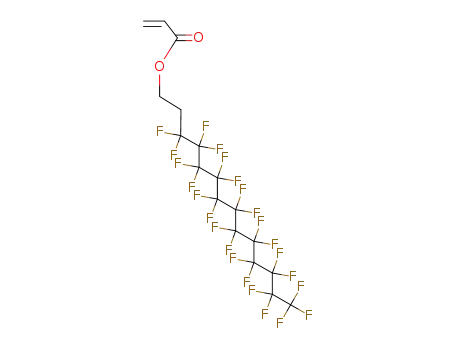
- 34395-24-9
3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,14,14,14-pentacosafluoro-tetradecyl acrylate
-
-
1H,1H,2H-perfluoro-1-tetradecene
-
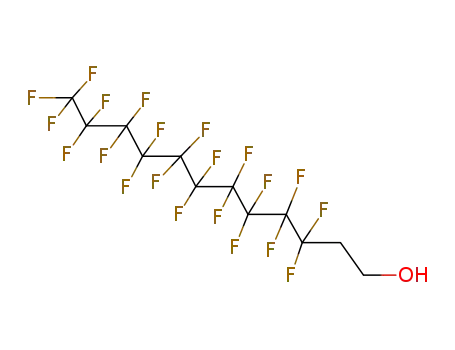
- 647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol
-
- 865-86-1
2-(perfluorodecyl)ethanol
-
- 25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene
-
- 21652-58-4
1H,1H,2H-perfluorodecene
-
- 39239-77-5
1H,1H,2H,2H-perfluorobutadecan-1-ol
-
- 678-39-7
3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluoro-1-decanol
-
- 30389-25-4
3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,12-henicosafluorododec-1-ene
-
- 27905-45-9
1H,1H,2H,2H-heptadecafluorodecyl acrylate
-
- 17527-29-6
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl 2-propenoate
-
- 17741-60-5
1,1,2,2-Tetrahydroperfluorododecyl acrylate
Conditions
| Conditions |
Yield |
|
polytetrafluoroethylene; Pentafluoroethyl iodide; copper catalyst; at 80 ℃; under 6000.6 Torr;
ethene; copper catalyst; at 100 ℃; for 3h; under 2250.23 Torr;
potassium acrylate; With 4-methoxy-phenol; hydroquinone; In tert-butyl alcohol; at 180 - 190 ℃; for 6h; Product distribution / selectivity;
|
|
25291-17-2 Upstream products
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane
-
123-39-7
N-Methylformamide
-
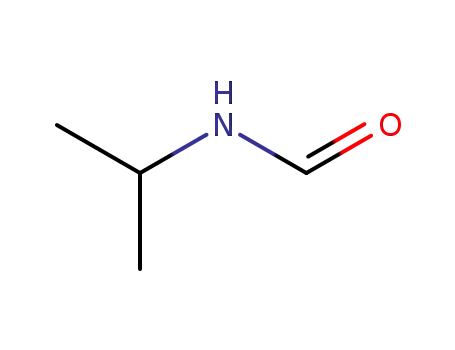
16741-46-1
N-isopropylformamide
-
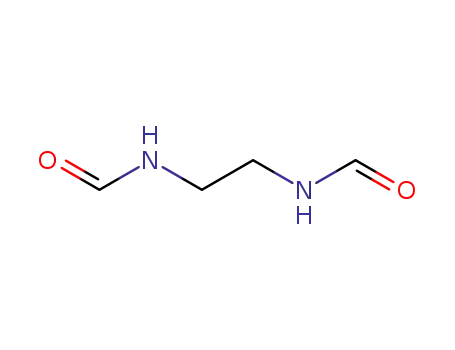
4938-92-5
N-(2-formylaminoethyl)formamide
25291-17-2 Downstream products
-
51249-62-8
1,2-Dibrom-1H,1H,2H-perfluorooctane
-
73609-36-6
(1H,1H,2H,2H-tridecafluoro-octyl)(methyl)dichlorosilane
-
74-85-1
ethene
-
56523-43-4
7H,8H-hexacosafluoro-tetradec-7-ene