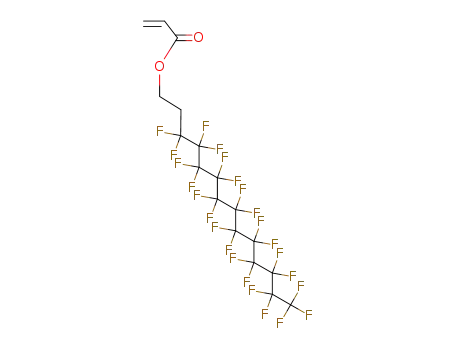
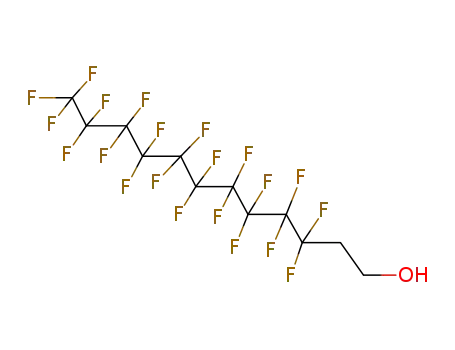
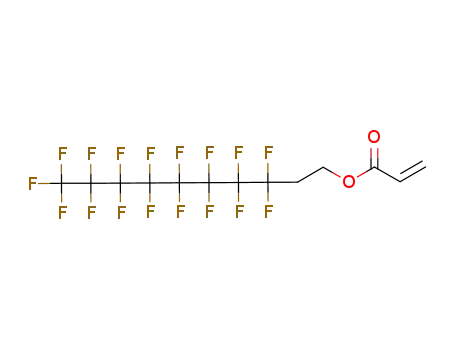
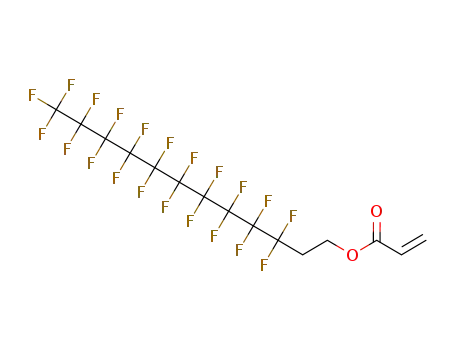
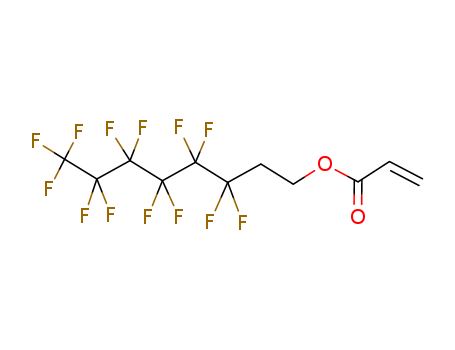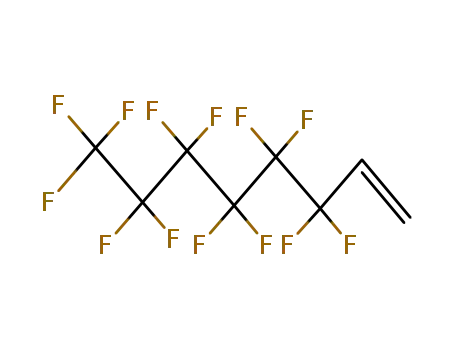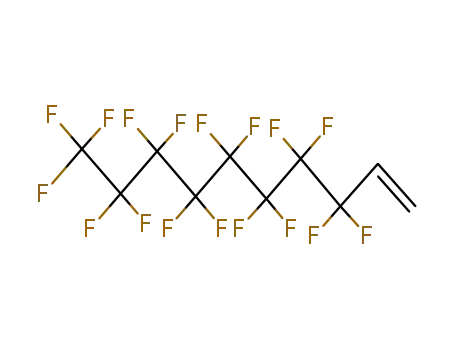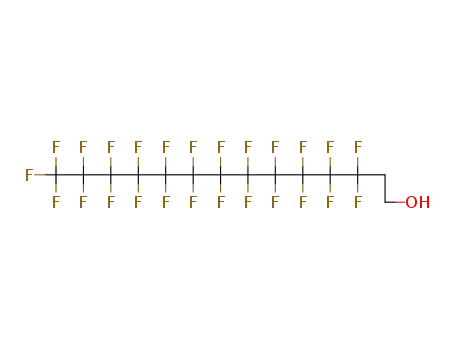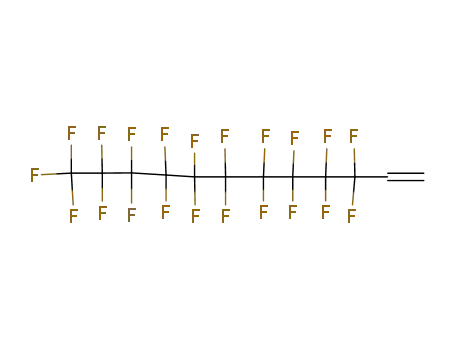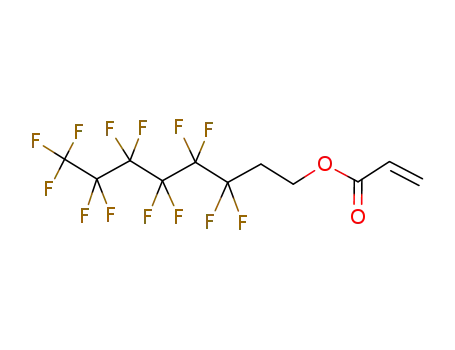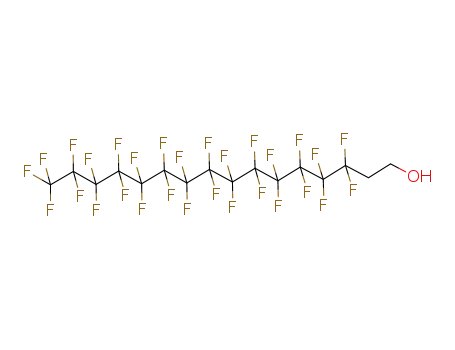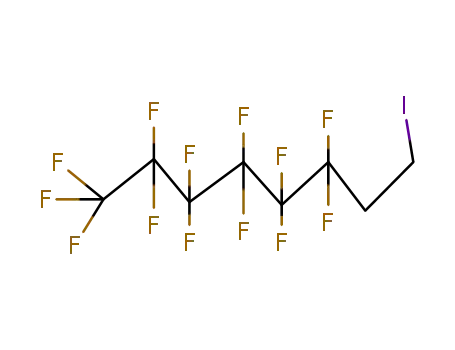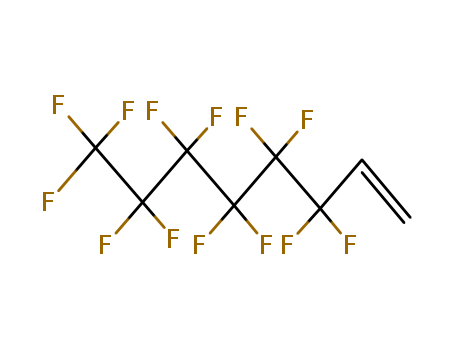Sale Factory Supply 1H,1H,2H,2H-Perfluorooctyl acrylate 17527-29-6 Cheapest Price
- Molecular Formula:C11H7F13O2
- Molecular Weight:418.155
- Vapor Pressure:0.332mmHg at 25°C
- Refractive Index:n20/D 1.338(lit.)
- Boiling Point:200 °C at 760 mmHg
- Flash Point:73 °C
- PSA:26.30000
- Density:1.494 g/cm3
- LogP:4.84450
1H,1H,2H,2H-Perfluorooctyl acrylate(Cas 17527-29-6) Usage
|
Chemical Properties
|
Colorless liquid |
|
Uses
|
1H,1H,2H,2H-Perfluorooctyl Acrylate is a semi-volatile fluorinated organic compound found in spring-time polluted Asian and western US air masses. |
InChI:InChI=1/C11H7F13O2/c1-2-5(25)26-4-3-6(12,13)7(14,15)8(16,17)9(18,19)10(20,21)11(22,23)24/h2H,1,3-4H2
17527-29-6 Relevant articles
Synthesis and characterization of semifluorinated polymers via group transfer polymerization
Krupers, Maarten J.,Moeller, Martin
, p. 119 - 124 (1997)
Group transfer polymerization (GTP) was ...
Cross-polarization for a fluoropolymer involving multiple spin baths of abundant nuclei
Hazendonk, Paul,Harris, Robin K.,Galli, Giancarlo,Pizzanelli, Silvia
, p. 507 - 513 (2002)
The thermodynamic theory of cross-polari...
-
Boutevin,Rigal,Rousseau,Bosc
, p. 47 - 73 (1988)
Several fluorinated and chlorofluorinate...
METHOD FOR PRODUCING FLUORINE-CONTAINING (METH) ACRYLATE
-
Paragraph 0053-0056; 0059, (2021/03/13)
To provide a method for producing fluori...
Method for preparing fluorinated acrylate from fluorinated alcohol (by machine translation)
-
Paragraph 0035-0038; 0042-0044; 0054-0056, (2020/07/21)
Under the presence of a self-made fluori...
Environmentally friendly preparation method of fluorine-containing acrylate
-
Paragraph 0025, (2019/01/06)
The invention relates to the technical f...
PRODUCTION METHOD OF FLUORINE-CONTAINING (METH)ACRYLIC ACID ESTER
-
Paragraph 0039, (2016/10/07)
PROBLEM TO BE SOLVED: To provide a metho...
17527-29-6 Process route
-
-
116-14-3,82785-14-6
polytetrafluoroethylene
-
-
354-64-3
Pentafluoroethyl iodide
-
-
10192-85-5
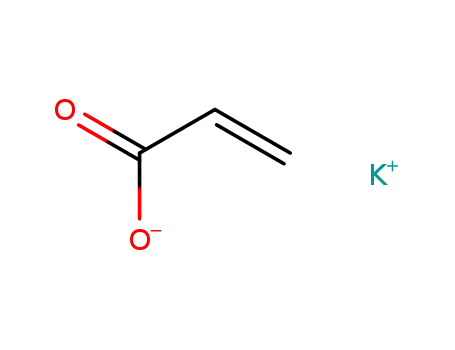
potassium acrylate
-
-
34395-24-9
3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,14,14,14-pentacosafluoro-tetradecyl acrylate
-
-
1H,1H,2H-perfluoro-1-tetradecene
-
-
647-42-7
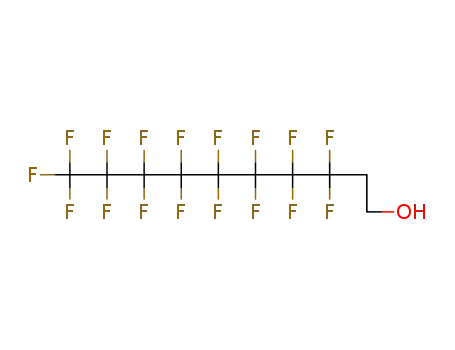
1H,1H,2H,2H-tridecafluoro-n-octanol
-
-
865-86-1
2-(perfluorodecyl)ethanol
-
-
25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene
-
-
21652-58-4
1H,1H,2H-perfluorodecene
-
-
39239-77-5
1H,1H,2H,2H-perfluorobutadecan-1-ol
-
-
678-39-7
3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluoro-1-decanol
-
-
30389-25-4
3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,12-henicosafluorododec-1-ene
-
-
27905-45-9
1H,1H,2H,2H-heptadecafluorodecyl acrylate
-
-
17527-29-6
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl 2-propenoate
-
-
17741-60-5
1,1,2,2-Tetrahydroperfluorododecyl acrylate
Conditions
| Conditions |
Yield |
|
polytetrafluoroethylene; Pentafluoroethyl iodide;
copper catalyst;
at 80 ℃;
under 6000.6 Torr;
ethene;
copper catalyst;
at 100 ℃;
for 3h;
under 2250.23 Torr;
potassium acrylate;
With
4-methoxy-phenol; hydroquinone;
In
tert-butyl alcohol;
at 180 - 190 ℃;
for 6h;
Product distribution / selectivity;
|
|
-
-
116-14-3,82785-14-6
polytetrafluoroethylene
-
-
354-64-3
Pentafluoroethyl iodide
-
-
10192-85-5
potassium acrylate
-
-
34395-24-9
3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,14,14,14-pentacosafluoro-tetradecyl acrylate
-
-
1H,1H,2H-perfluoro-1-tetradecene
-
-
60699-51-6
1H,1H,2H,2H-perfluorohexadecan-1-ol
-
-
34362-49-7
C14F29CH2CH2OCOCH=CH2
-
-
65104-64-5
C18F37CH2CH2OCOCH=CH2
-
-
865-86-1
2-(perfluorodecyl)ethanol
-
-
21652-58-4
1H,1H,2H-perfluorodecene
-
-
39239-77-5
1H,1H,2H,2H-perfluorobutadecan-1-ol
-
-
678-39-7
3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluoro-1-decanol
-
-
30389-25-4
3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,12-henicosafluorododec-1-ene
-
-
27905-45-9
1H,1H,2H,2H-heptadecafluorodecyl acrylate
-
-
17527-29-6
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl 2-propenoate
-
-
17741-60-5
1,1,2,2-Tetrahydroperfluorododecyl acrylate
Conditions
| Conditions |
Yield |
|
polytetrafluoroethylene; Pentafluoroethyl iodide;
copper catalyst;
at 80 ℃;
under 6000.6 Torr;
ethene;
copper catalyst;
at 100 ℃;
for 3h;
under 2250.23 Torr;
potassium acrylate;
With
4-methoxy-phenol; hydroquinone;
In
tert-butyl alcohol;
at 180 - 190 ℃;
for 6h;
Product distribution / selectivity;
|
|
17527-29-6 Upstream products
-
647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol
-
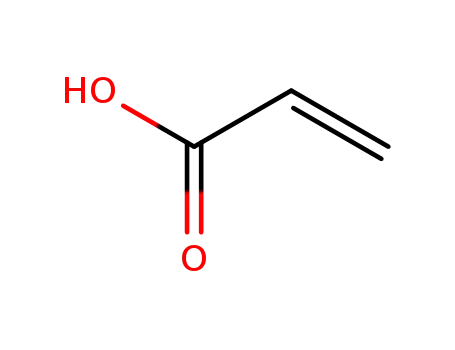
79-10-7
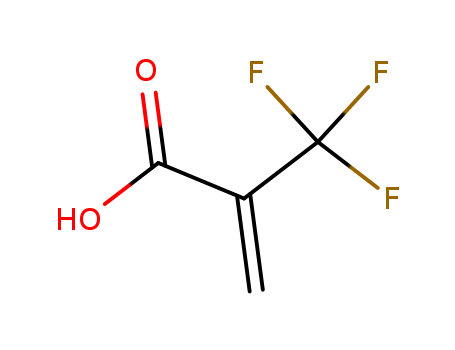
acrylic acid
-
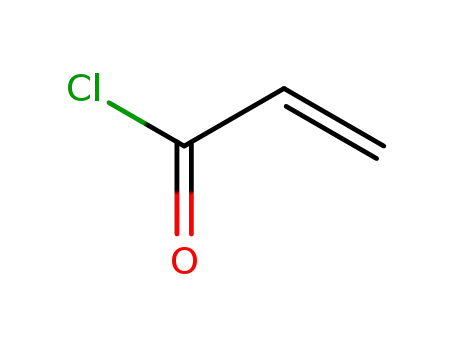
814-68-6
acryloyl chloride
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane