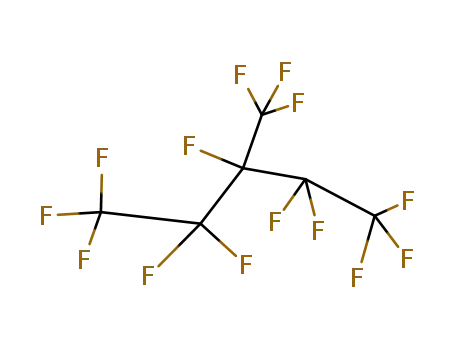
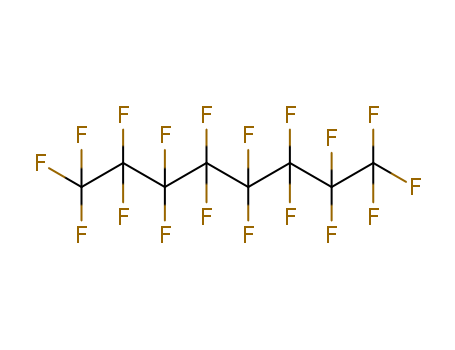Top Purity Quality Manufacturer Supply PERFLUOROHEXANE 355-42-0 Low Price
- Molecular Formula:C6F14
- Molecular Weight:338.044
- Appearance/Colour:colourless liquid
- Vapor Pressure:228mmHg at 25°C
- Melting Point:-4 °C(lit.)
- Refractive Index:n20/D 1.252(lit.)
- Boiling Point:57.8 °C at 760 mmHg
- Flash Point:1.2 °C
- PSA:0.00000
- Density:1.656 g/cm3
- LogP:4.65220
PERFLUOROHEXANE(Cas 355-42-0) Usage
|
Chemical Properties
|
colourless liquid |
|
Uses
|
In the electronics industry as a coolant and test bath medium. Non-toxic, non-ozone-depleting, inert reaction medium. |
|
Application
|
Perfluorohexane uses and applications include: dispersant for lubricants, mold release agents, protective coatings; reaction media for polymerizations, purification, separation processes; solvent for removal of halogenated lubricants, oils, greases; carrier for halogenated material; for quick-cooling or freezing foods; heat-transfer fluid.
Because it is biologically inert and chemically stable, perfluorohexane has attracted attention in medicine. Like other fluorocarbons, perfluorohexane dissolves gases, including oxygen from the air, to a higher concentration than ordinary organic solvents. This effect is attributed to the weak intermolecular forces between perfluorohexane molecules, which allows "space" for gas molecules to partition into the liquid. Animals can be submerged in a bath of perfluorohexane without drowning, as there is sufficient oxygen available in the solvent to allow respiration to continue. This effect has led to the experimental use of perfluorohexane in treating burn victims, as their lungs can be filled with either perfluorohexane vapor or in extreme cases liquid perfluorohexane, allowing breathing to continue without the problems normally seen with pulmonary edema that sometimes occur when the inside of the lungs have been burnt e.g. by inhalation of hot smoke. |
|
General Description
|
Tetradecafluorohexane in the gas phase reacts spontaneously with lithium amalgam, to give a solid and intimate mixture of lithium fluoride and elemental polymeric carbon with a small amount of superstoichiometric lithium. |
|
Purification Methods
|
Purify the fluorohexane by fractional freezing. The methods described for perfluoroheptane should be applicable here. [Beilstein 1 IV 348.] |
InChI:InChI=1/C6F14/c7-1(8,3(11,12)5(15,16)17)2(9,10)4(13,14)6(18,19)20
355-42-0 Relevant articles
THE CHEMISTRY OF DIACYL PEROXIDES - VIII. THE REACTIONS BETWEEN POLYFLUORODIACYL PEROXIDES AND 2-NITRO-2-NITROSOPROPANE--GENERATION OF BIS(POLYFLUOROALKYL) NITROXIDES
Zhao, Cheng-Xue,Chen, Guo-Fei,Jiang, Xi-Kui,Wang, Xian-Shan
, p. 597 - 606 (1987)
Thermal decomposition of 2-nitro-2-nitro...
-
Hauptschein et al.
, p. 6248,6249,6251 (1957)
-
Synthesis method of straight-chain perfluoroalkanes
-
Paragraph 0018; 0019; 0027, (2019/05/08)
The invention relates to a synthesis met...
A preparation method of the whole fluorine hexanone (by machine translation)
-
Paragraph 0025; 0026; 0027; 0028, (2019/01/04)
The invention discloses a full-fluorine ...
Decomposition characteristics of C5F10O/air mixture as substitutes for SF6 to reduce global warming
Li, Yi,Zhang, Xiaoxing,Xiao, Song,Chen, Qi,Wang, Dibo
, p. 65 - 72 (2018/02/14)
Sulfur hexafluoride (SF6) is widely used...
Preparation method for perfluoroalkane
-
Paragraph 0026; 0027, (2016/11/28)
The invention discloses a preparation me...
355-42-0 Process route
-
-
922-61-2
3-methyl-2-pentene
-
-
355-42-0
tetradecafluorohexane
-
-
355-04-4
perfluoroisohexane
-
-
865-71-4
perfluoro(3-methylpentane)
-
-
1805-22-7
perfluoro(2-methylcyclopentane)
-
-
112156-74-8
perfluoro(2,2-dimethylbutane)
Conditions
| Conditions |
Yield |
|
cobalt (III) fluoride;
at 360 ℃;
for 3h;
Product distribution;
|
7%
7%
61%
1%
24%
|
-
-
355-42-0
tetradecafluorohexane
-
-
355-04-4
perfluoroisohexane
-
-
865-71-4
perfluoro(3-methylpentane)
-
-
1805-22-7
perfluoro(2-methylcyclopentane)
Conditions
| Conditions |
Yield |
|
cobalt (III) fluoride;
at 360 ℃;
for 3h;
Further byproducts given;
|
11%
5%
7%
76%
|
355-42-0 Upstream products
-
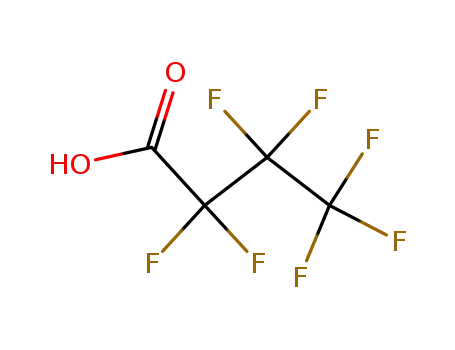
375-22-4
heptafluorobutyric Acid
-
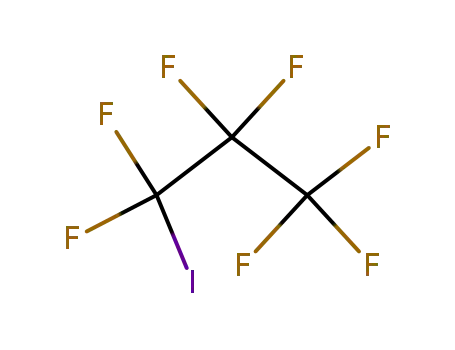
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane
-
110-54-3
hexane
-
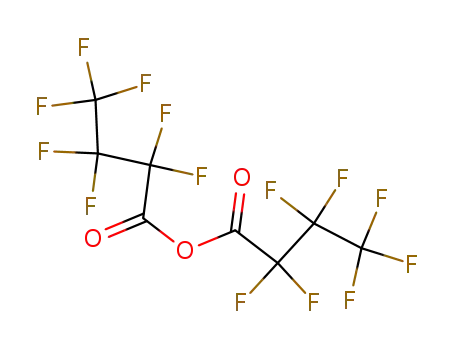
336-59-4
heptafluorobutyric anhydride
355-42-0 Downstream products
-
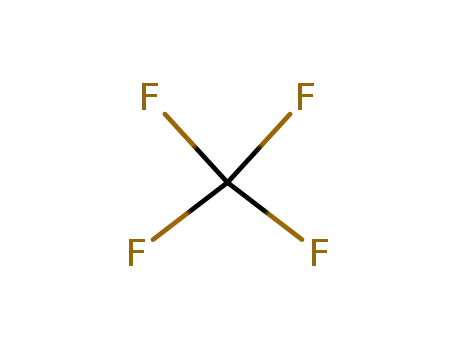
75-73-0
carbon tetrafluoride
-
75-72-9
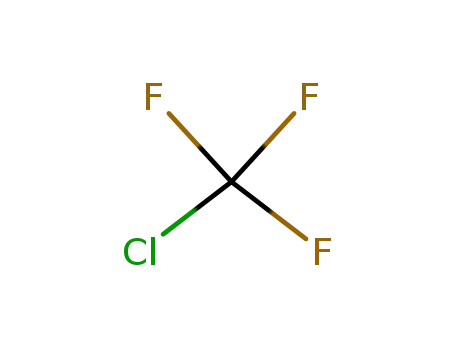
chlorotrifluoromethane
-
90499-30-2
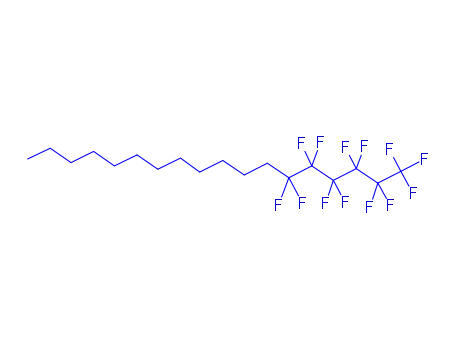
1,1,1,2,2,3,3,4,4,5,5,6,6-tridecafluoro-octadecane
-
174861-40-6
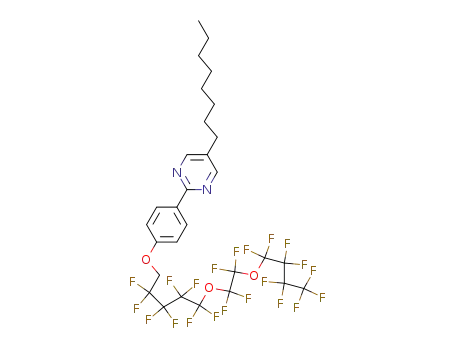
5-Octyl-2-(4-(1,1-dihydroperfluoro-5-(2-butoxyethoxy) pentoxy)phenyl)pyrimidine