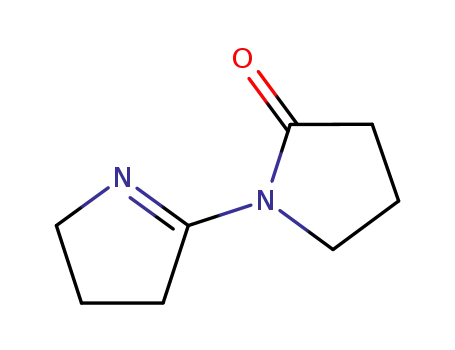
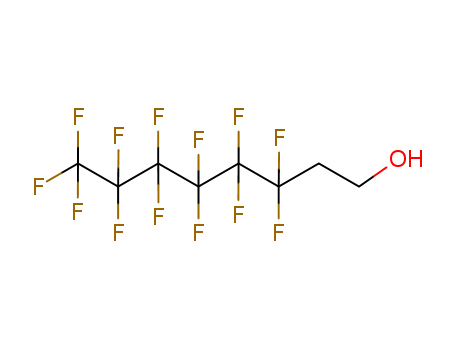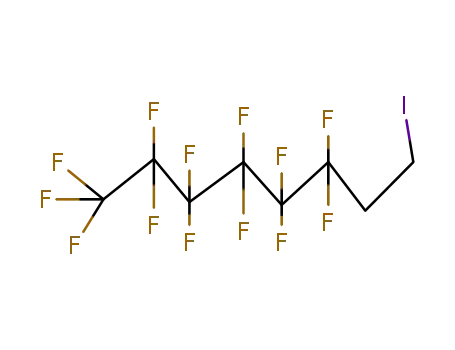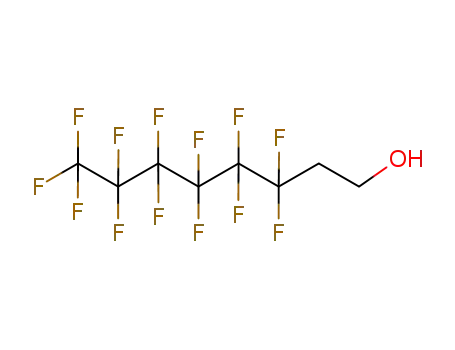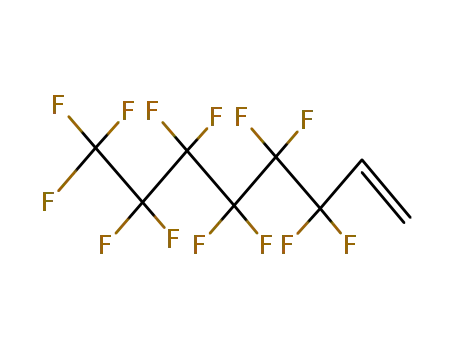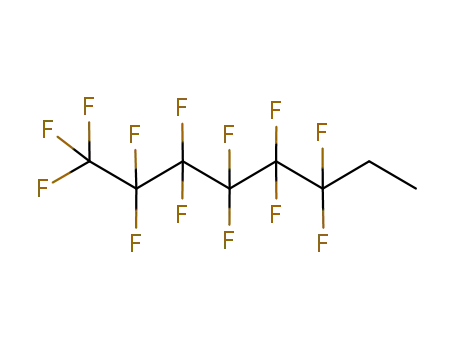Chinese Manufacturer Supply 99% Pure 1H,1H,2H,2H-PERFLUORO-1-OCTANOL 647-42-7 In Bulk Supply
- Molecular Formula:C8H5F13O
- Molecular Weight:364.106
- Appearance/Colour:Colorless liquid
- Vapor Pressure:0.382mmHg at 25°C
- Refractive Index:n20/D 1.313(lit.)
- Boiling Point:174.1 °C at 760 mmHg
- PKA:14.26±0.10(Predicted)
- Flash Point:91.667 °C
- PSA:20.23000
- Density:1.589 g/cm3
- LogP:4.10760
3,3,4,4,5,5,6,6,7,7,8,8,8-Tridecafluoro-1-octanol(Cas 647-42-7) Usage
|
Chemical Properties
|
CLEAR COLOURLESS LIQUID
|
|
Uses
|
1H,1H,2H,2H-Perfluoro-1-octanol is a material used to improve nanotube composites. It is also used in the synthesis of a recyclable fluorous hydrazine carbothioate compound with NCS to catalyz e the acetalization of aldehydes.
|
|
General Description
|
1H,1H,2H,2H-Perfluoro-1-octanol is a fluorotelomer alcohol.
|
InChI:InChI=1/C8H5F13O/c9-3(10,1-2-22)4(11,12)5(13,14)6(15,16)7(17,18)8(19,20)21/h22H,1-2H2
647-42-7 Relevant articles
Further evidence on the importance of fluorous-fluorous interactions in supramolecular chemistry: A combined structural and computational study
Omorodion, Harrison,Twamley, Brendan,Platts, James A.,Baker, Robert J.
, p. 2835 - 2841 (2015)
The solid-state structures of CF3(CF2)5C...
Environmentally friendly preparation method of fluorine-containing acrylate
-
Paragraph 0024; 0027, (2019/01/06)
The invention relates to the technical f...
Method for preparing perfluoroalkyl ethanol
-
Paragraph 0026-0032, (2017/11/29)
The invention discloses a method for pre...
Perfluoroalkyl ethyl alcohols via perfluoroalkyl acetaldehydes
Peng, Sheng,Moloy, Kenneth G.
, p. 7 - 10 (2017/08/04)
A new route to commercially important pe...
Method for preparing perfluoroalkyl alcohol from perfluoroalkyl ethylene
-
Paragraph 0026; 0028; 0029, (2017/04/03)
The invention discloses a method for pre...
647-42-7 Process route
-
- 2043-57-4,71215-70-8
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane
-
- 647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol
-
- 25291-17-2
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooct-1-ene
-
- 80793-17-5
(perfluoro-n-hexyl)ethane
Conditions
| Conditions |
Yield |
|
With water; lithium chloride; In N,N-dimethyl-formamide; Product distribution; effect of quantity of water and current on electroreduction;
|
80 % Spectr.
5 % Spectr.
15 % Spectr. |
-
- 155939-24-5
3,4-Dihydro-5-<(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluoro)octyloxy>-2H-pyrrole
-
- 647-42-7
1H,1H,2H,2H-tridecafluoro-n-octanol
-
- 7060-52-8
1-(1-pyrrolin-2-yl)-2-pyrrolidinone
Conditions
| Conditions |
Yield |
|
With hydrogenchloride; In chloroform-d1; acetone; at 25 - 30 ℃; for 3h; Product distribution; other duration;
|
6.40 % Chromat.
0.147 % Chromat.
2.66 % Chromat. |
647-42-7 Upstream products
-
123-39-7
N-Methylformamide
-
2043-57-4
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane
-
16741-46-1
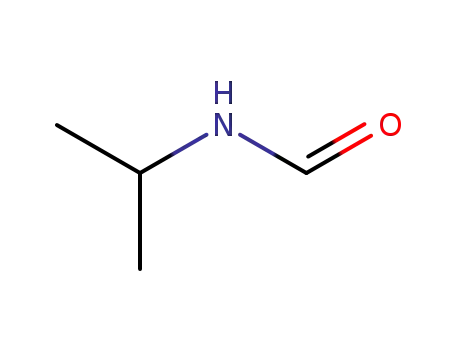
N-isopropylformamide
-
4938-92-5
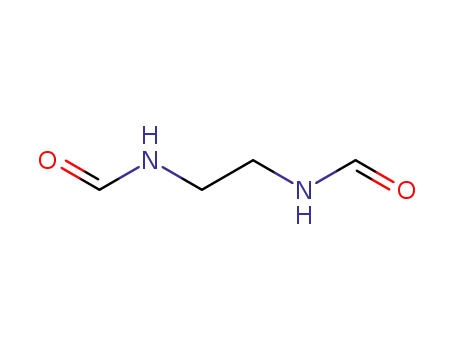
N-(2-formylaminoethyl)formamide
647-42-7 Downstream products
-
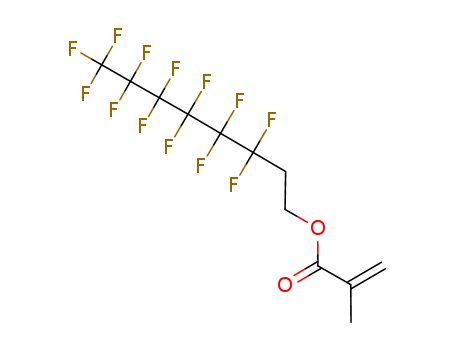
2144-53-8
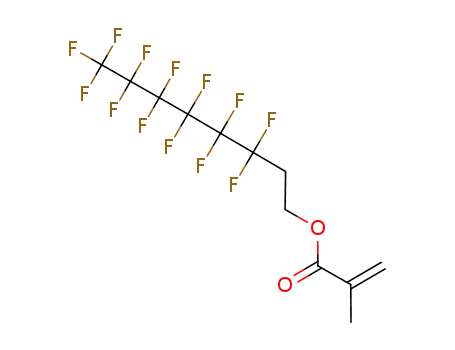
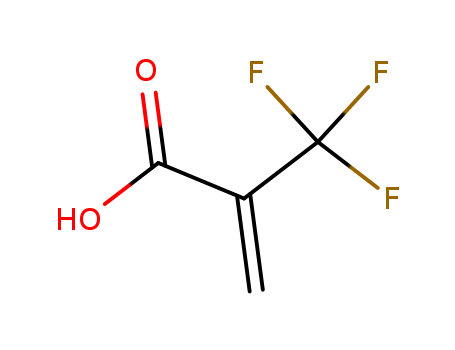
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl 2-methylpropenoate
-
141564-12-7
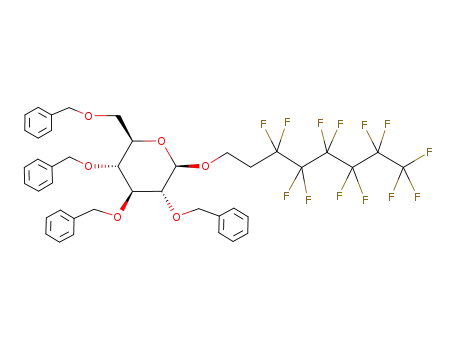
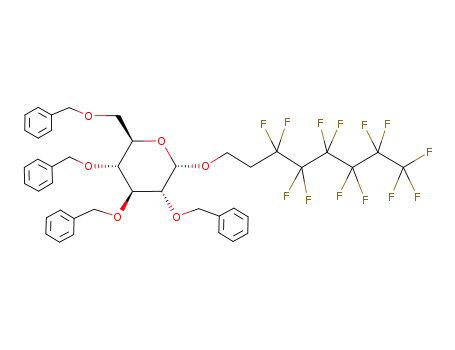
3',3',4',4',5',5',6',6',7',7',8',8',8'-tridecafluorooctyl 2,3,4,6-tetra-O-benzyl-β-D-glucopyranoside
-
141564-11-6
3',3',4',4',5',5',6',6',7',7',8',8',8'-tridecafluorooctyl 2,3,4,6-tetra-O-benzyl-α-D-glucopyranoside
-
64018-25-3
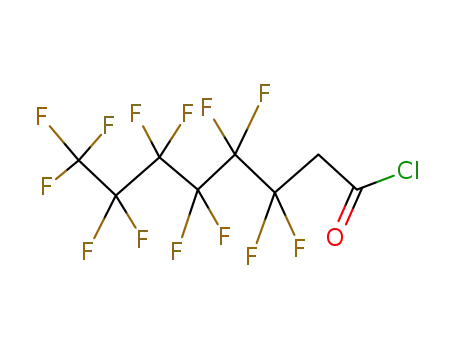
3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctanoyl chloride