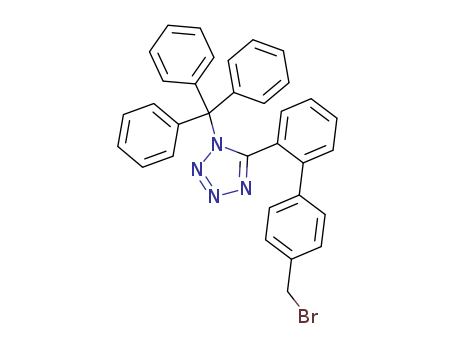
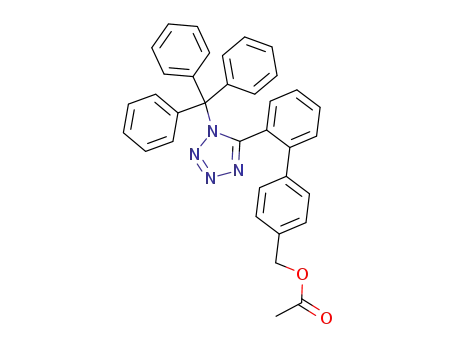
Hot Sale Factory Supply N-(Triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl-)tetrazole 124750-51-2 Lowest Price
- Molecular Formula:C33H25BrN4
- Molecular Weight:557.492
- Appearance/Colour:off-white solid
- Melting Point:152-155 °C
- Boiling Point:718.441 °C at 760 mmHg
- PKA:0.29±0.10(Predicted)
- Flash Point:388.299 °C
- PSA:43.60000
- Density:1.282 g/cm3
- LogP:7.74220
124750-51-2 Relevant articles
Method for synthesizing high-purity sartan side chain TTBB
-
Paragraph 0020; 0043-0045, (2018/06/15)
The invention discloses a method for syn...
Method for tubular reaction preparation of substituted benzylically brominated methyl biphenyl and reaction device of method
-
Paragraph 0022-0029, (2018/06/15)
The invention discloses a method for tub...
Bacterial Peptide Deformylase Inhibition of Tetrazole-Substituted Biaryl Acid Analogs: Synthesis, Biological Evaluations, and Molecular Docking Study
Khan, Firoz A. Kalam,Patil, Rajendra H.,Patil, Manjiri,Arote, Rohidas,Shinde, Devanand B.,Sangshetti, Jaiprakash N.
, p. 934 - 943 (2016/12/09)
The synthesis and screening of tetrazole...
1,(3,)5-substituted imidazoles, useful in the treatment of hypertension and methods for their preparation
-
Paragraph 0219, (2016/03/04)
The present invention provides novel 1,5...
124750-51-2 Process route
-
-
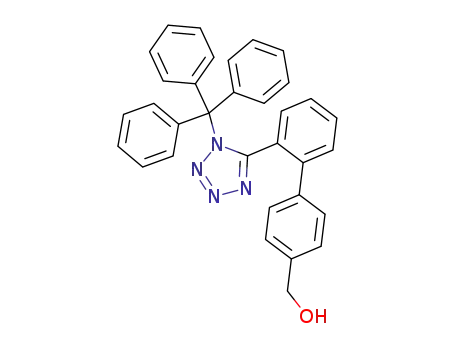
154709-18-9
4'-hydroxymethyl-2-(N-trityl-1H-tetrazol-5-yl)biphenyl
-
-
124750-51-2
N-(triephenylmethyl)-5-<4'-(bromomethyl)-biphenyl-2-yl>tetrazole
Conditions
| Conditions |
Yield |
|
With
phosphorus tribromide;
In
dichloromethane;
at 10 - 20 ℃;
for 1h;
|
95.07%
|
|
With
sulfuric acid; potassium bromide;
In
N,N-dimethyl acetamide;
at 10 - 25 ℃;
for 7h;
Reagent/catalyst;
Solvent;
|
81.4%
|
-
-
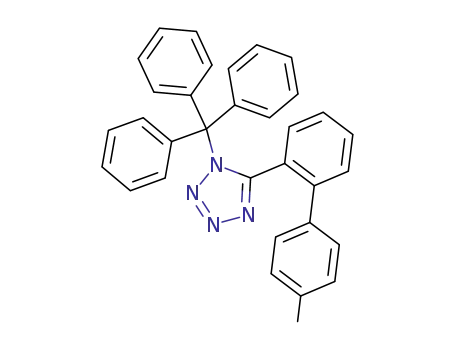
124750-53-4
N-triphenylmethyl-5-(4'-methylbiphenyl-2-yl)tetrazole
-
-
124750-51-2
N-(triephenylmethyl)-5-<4'-(bromomethyl)-biphenyl-2-yl>tetrazole
Conditions
| Conditions |
Yield |
|
With
bromine;
In
hexane; water;
at 69 ℃;
for 0.433333h;
Temperature;
Solvent;
Irradiation;
Flow reactor;
|
95.2%
|
|
With
sodium bromate; sodium hydrogensulfite;
In
water; 1,2-dichloro-ethane;
at 20 ℃;
Product distribution / selectivity;
|
90%
|
|
With
N-Bromosuccinimide; dihydrogen peroxide;
In
dichloromethane;
Reflux;
|
88%
|
|
N-triphenylmethyl-5-(4'-methylbiphenyl-2-yl)tetrazole;
With
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; 2,2'-azobis(isobutyronitrile);
In
chlorobenzene;
at 80 ℃;
With
potassium carbonate; phosphonic acid diethyl ester;
In
tetrahydrofuran;
Heating;
|
87%
|
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
tetrachloromethane;
|
85%
|
|
With
N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile);
In
cyclohexane;
for 12h;
Heating;
|
83.8%
|
|
With
N-Bromosuccinimide; dihydrogen peroxide;
In
dichloromethane;
Reflux;
|
80%
|
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
tetrachloromethane;
for 3h;
Reflux;
|
20%
|
|
With
N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile);
In
tetrachloromethane;
for 3h;
Yield given;
Heating;
|
|
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
tetrachloromethane;
|
|
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
tetrachloromethane;
|
|
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
dichloromethane;
for 15h;
Heating / reflux;
|
|
|
With
N-Bromosuccinimide;
dibenzoyl peroxide;
In
dichloromethane;
for 15h;
Heating / reflux;
|
|
|
With
N-Bromosuccinimide;
dibenzoyl peroxide;
In
dichloromethane;
for 15h;
Heating / reflux;
|
|
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
dichloromethane;
for 15h;
Heating / reflux;
|
|
|
With
N-Bromosuccinimide;
dibenzoyl peroxide;
In
dichloromethane;
for 15h;
Reflux;
|
|
|
With
N-Bromosuccinimide;
dibenzoyl peroxide;
In
dichloromethane;
for 15h;
Inert atmosphere;
Reflux;
|
|
|
With
bromine; acetic acid;
|
|
|
With
N-Bromosuccinimide;
In
tetrachloromethane;
|
|
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
tetrachloromethane;
|
|
124750-51-2 Upstream products
124750-51-2 Downstream products