| Conditions |
Yield |
|
Multi-step reaction with 5 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.33 h / 20 °C / Inert atmosphere
2.2: 0.17 h / -78 °C / Inert atmosphere
3.1: dichloromethane / 0.17 h / -78 °C / Inert atmosphere
3.2: 1 h / -78 °C / Inert atmosphere
4.1: boron trichloride / dichloromethane / 1 h / -78 - -20 °C / Inert atmosphere
5.1: tert-butylmagnesium chloride / tetrahydrofuran; N,N-dimethyl-formamide / 2 h / 20 °C / Inert atmosphere
With chloro-trimethyl-silane; tert-butylmagnesium chloride; acetic anhydride; boron trichloride; dimethyl sulfoxide; In tetrahydrofuran; dichloromethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.33 h / 20 °C / Inert atmosphere
2.2: 0.17 h / -78 °C / Inert atmosphere
3.1: dichloromethane / 0.17 h / -78 °C / Inert atmosphere
3.2: 1 h / -78 °C / Inert atmosphere
4.1: boron trichloride / dichloromethane / 1 h / -78 - -20 °C / Inert atmosphere
5.1: magnesium chloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl acetamide / 5 h / 30 °C
With chloro-trimethyl-silane; acetic anhydride; boron trichloride; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine; magnesium chloride; In tetrahydrofuran; dichloromethane; N,N-dimethyl acetamide;
|
|
|
Multi-step reaction with 5 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.33 h / 20 °C / Inert atmosphere
2.2: 0.17 h / -78 °C / Inert atmosphere
3.1: dichloromethane / 0.17 h / -78 °C / Inert atmosphere
3.2: 1 h / -78 °C / Inert atmosphere
4.1: boron trichloride / dichloromethane / 1 h / -78 - -20 °C / Inert atmosphere
5.1: magnesium chloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl acetamide / 6 h / 30 °C
With chloro-trimethyl-silane; acetic anhydride; boron trichloride; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine; magnesium chloride; In tetrahydrofuran; dichloromethane; N,N-dimethyl acetamide;
|
|
|
Multi-step reaction with 5 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.33 h / 20 °C / Inert atmosphere
2.2: 0.17 h / -78 °C / Inert atmosphere
3.1: trimethylsilyl trifluoromethanesulfonate; trifluoroacetic acid / dichloromethane / 0.17 h / -40 - -25 °C
4.1: boron trichloride / dichloromethane / 1 h / -20 - -15 °C
5.1: tert-butylmagnesium chloride / tetrahydrofuran; N,N-dimethyl-formamide / 2 h / 20 °C / Inert atmosphere
With chloro-trimethyl-silane; trimethylsilyl trifluoromethanesulfonate; tert-butylmagnesium chloride; acetic anhydride; boron trichloride; dimethyl sulfoxide; trifluoroacetic acid; In tetrahydrofuran; dichloromethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.33 h / 20 °C / Inert atmosphere
2.2: 0.17 h / -78 °C / Inert atmosphere
3.1: trimethylsilyl trifluoromethanesulfonate; trifluoroacetic acid / dichloromethane / 0.17 h / -40 - -25 °C
4.1: boron trichloride / dichloromethane / 1 h / -20 - -15 °C
5.1: magnesium chloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl acetamide / 5 h / 30 °C
With chloro-trimethyl-silane; trimethylsilyl trifluoromethanesulfonate; acetic anhydride; boron trichloride; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine; trifluoroacetic acid; magnesium chloride; In tetrahydrofuran; dichloromethane; N,N-dimethyl acetamide;
|
|
|
Multi-step reaction with 5 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.33 h / 20 °C / Inert atmosphere
2.2: 0.17 h / -78 °C / Inert atmosphere
3.1: trimethylsilyl trifluoromethanesulfonate; trifluoroacetic acid / dichloromethane / 0.17 h / -40 - -25 °C
4.1: boron trichloride / dichloromethane / 1 h / -20 - -15 °C
5.1: magnesium chloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl acetamide / 6 h / 30 °C
With chloro-trimethyl-silane; trimethylsilyl trifluoromethanesulfonate; acetic anhydride; boron trichloride; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine; trifluoroacetic acid; magnesium chloride; In tetrahydrofuran; dichloromethane; N,N-dimethyl acetamide;
|
|
|
Multi-step reaction with 5 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.5 h / 0 °C
2.2: 0.58 h / 0 - 5 °C
3.1: dichloromethane / 0.17 h / -78 °C / Inert atmosphere
3.2: 1 h / -78 °C / Inert atmosphere
4.1: boron trichloride / dichloromethane / 1 h / -78 - -20 °C / Inert atmosphere
5.1: tert-butylmagnesium chloride / tetrahydrofuran; N,N-dimethyl-formamide / 2 h / 20 °C / Inert atmosphere
With chloro-trimethyl-silane; tert-butylmagnesium chloride; acetic anhydride; boron trichloride; dimethyl sulfoxide; In tetrahydrofuran; dichloromethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.5 h / 0 °C
2.2: 0.58 h / 0 - 5 °C
3.1: dichloromethane / 0.17 h / -78 °C / Inert atmosphere
3.2: 1 h / -78 °C / Inert atmosphere
4.1: boron trichloride / dichloromethane / 1 h / -78 - -20 °C / Inert atmosphere
5.1: magnesium chloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl acetamide / 5 h / 30 °C
With chloro-trimethyl-silane; acetic anhydride; boron trichloride; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine; magnesium chloride; In tetrahydrofuran; dichloromethane; N,N-dimethyl acetamide;
|
|
|
Multi-step reaction with 5 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.5 h / 0 °C
2.2: 0.58 h / 0 - 5 °C
3.1: dichloromethane / 0.17 h / -78 °C / Inert atmosphere
3.2: 1 h / -78 °C / Inert atmosphere
4.1: boron trichloride / dichloromethane / 1 h / -78 - -20 °C / Inert atmosphere
5.1: magnesium chloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl acetamide / 6 h / 30 °C
With chloro-trimethyl-silane; acetic anhydride; boron trichloride; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine; magnesium chloride; In tetrahydrofuran; dichloromethane; N,N-dimethyl acetamide;
|
|
|
Multi-step reaction with 5 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.5 h / 0 °C
2.2: 0.58 h / 0 - 5 °C
3.1: trimethylsilyl trifluoromethanesulfonate; trifluoroacetic acid / dichloromethane / 0.17 h / -40 - -25 °C
4.1: boron trichloride / dichloromethane / 1 h / -20 - -15 °C
5.1: tert-butylmagnesium chloride / tetrahydrofuran; N,N-dimethyl-formamide / 2 h / 20 °C / Inert atmosphere
With chloro-trimethyl-silane; trimethylsilyl trifluoromethanesulfonate; tert-butylmagnesium chloride; acetic anhydride; boron trichloride; dimethyl sulfoxide; trifluoroacetic acid; In tetrahydrofuran; dichloromethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.5 h / 0 °C
2.2: 0.58 h / 0 - 5 °C
3.1: trimethylsilyl trifluoromethanesulfonate; trifluoroacetic acid / dichloromethane / 0.17 h / -40 - -25 °C
4.1: boron trichloride / dichloromethane / 1 h / -20 - -15 °C
5.1: magnesium chloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl acetamide / 5 h / 30 °C
With chloro-trimethyl-silane; trimethylsilyl trifluoromethanesulfonate; acetic anhydride; boron trichloride; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine; trifluoroacetic acid; magnesium chloride; In tetrahydrofuran; dichloromethane; N,N-dimethyl acetamide;
|
|
|
Multi-step reaction with 5 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.5 h / 0 °C
2.2: 0.58 h / 0 - 5 °C
3.1: trimethylsilyl trifluoromethanesulfonate; trifluoroacetic acid / dichloromethane / 0.17 h / -40 - -25 °C
4.1: boron trichloride / dichloromethane / 1 h / -20 - -15 °C
5.1: magnesium chloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl acetamide / 6 h / 30 °C
With chloro-trimethyl-silane; trimethylsilyl trifluoromethanesulfonate; acetic anhydride; boron trichloride; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine; trifluoroacetic acid; magnesium chloride; In tetrahydrofuran; dichloromethane; N,N-dimethyl acetamide;
|
|
|
Multi-step reaction with 7 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.33 h / 20 °C / Inert atmosphere
2.2: 0.17 h / -78 °C / Inert atmosphere
3.1: dichloromethane / 0.17 h / -78 °C / Inert atmosphere
3.2: 1 h / -78 °C / Inert atmosphere
4.1: boron trichloride / dichloromethane / 1 h / -78 - -20 °C / Inert atmosphere
5.1: sulfuric acid / acetone / 0.5 h / 45 °C
6.1: magnesium chloride; N-ethyl-N,N-diisopropylamine / acetonitrile / 4 h / 20 °C
7.1: hydrogenchloride; water / tetrahydrofuran / 0 - 20 °C
With hydrogenchloride; chloro-trimethyl-silane; sulfuric acid; water; acetic anhydride; boron trichloride; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine; magnesium chloride; In tetrahydrofuran; dichloromethane; acetone; acetonitrile;
|
|
|
Multi-step reaction with 7 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.33 h / 20 °C / Inert atmosphere
2.2: 0.17 h / -78 °C / Inert atmosphere
3.1: trimethylsilyl trifluoromethanesulfonate; trifluoroacetic acid / dichloromethane / 0.17 h / -40 - -25 °C
4.1: boron trichloride / dichloromethane / 1 h / -20 - -15 °C
5.1: sulfuric acid / acetone / 0.5 h / 45 °C
6.1: magnesium chloride; N-ethyl-N,N-diisopropylamine / acetonitrile / 4 h / 20 °C
7.1: hydrogenchloride; water / tetrahydrofuran / 0 - 20 °C
With hydrogenchloride; chloro-trimethyl-silane; trimethylsilyl trifluoromethanesulfonate; sulfuric acid; water; acetic anhydride; boron trichloride; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine; trifluoroacetic acid; magnesium chloride; In tetrahydrofuran; dichloromethane; acetone; acetonitrile;
|
|
|
Multi-step reaction with 7 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.5 h / 0 °C
2.2: 0.58 h / 0 - 5 °C
3.1: dichloromethane / 0.17 h / -78 °C / Inert atmosphere
3.2: 1 h / -78 °C / Inert atmosphere
4.1: boron trichloride / dichloromethane / 1 h / -78 - -20 °C / Inert atmosphere
5.1: sulfuric acid / acetone / 0.5 h / 45 °C
6.1: magnesium chloride; N-ethyl-N,N-diisopropylamine / acetonitrile / 4 h / 20 °C
7.1: hydrogenchloride; water / tetrahydrofuran / 0 - 20 °C
With hydrogenchloride; chloro-trimethyl-silane; sulfuric acid; water; acetic anhydride; boron trichloride; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine; magnesium chloride; In tetrahydrofuran; dichloromethane; acetone; acetonitrile;
|
|
|
Multi-step reaction with 7 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.5 h / 0 °C
2.2: 0.58 h / 0 - 5 °C
3.1: trimethylsilyl trifluoromethanesulfonate; trifluoroacetic acid / dichloromethane / 0.17 h / -40 - -25 °C
4.1: boron trichloride / dichloromethane / 1 h / -20 - -15 °C
5.1: sulfuric acid / acetone / 0.5 h / 45 °C
6.1: magnesium chloride; N-ethyl-N,N-diisopropylamine / acetonitrile / 4 h / 20 °C
7.1: hydrogenchloride; water / tetrahydrofuran / 0 - 20 °C
With hydrogenchloride; chloro-trimethyl-silane; trimethylsilyl trifluoromethanesulfonate; sulfuric acid; water; acetic anhydride; boron trichloride; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine; trifluoroacetic acid; magnesium chloride; In tetrahydrofuran; dichloromethane; acetone; acetonitrile;
|
|
|
Multi-step reaction with 8 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.33 h / 20 °C / Inert atmosphere
2.2: 0.17 h / -78 °C / Inert atmosphere
3.1: dichloromethane / 0.17 h / -78 °C / Inert atmosphere
3.2: 1 h / -78 °C / Inert atmosphere
4.1: boron trichloride / dichloromethane / 1 h / -78 - -20 °C / Inert atmosphere
5.1: acetone / 0.5 h / 20 °C
6.1: potassium carbonate / water; ethyl acetate
7.1: magnesium chloride; N-ethyl-N,N-diisopropylamine / acetonitrile / 4 h / 20 °C
8.1: hydrogenchloride; water / tetrahydrofuran / 0 - 20 °C
With hydrogenchloride; chloro-trimethyl-silane; water; acetic anhydride; boron trichloride; potassium carbonate; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine; magnesium chloride; In tetrahydrofuran; dichloromethane; water; ethyl acetate; acetone; acetonitrile;
|
|
|
Multi-step reaction with 8 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.33 h / 20 °C / Inert atmosphere
2.2: 0.17 h / -78 °C / Inert atmosphere
3.1: trimethylsilyl trifluoromethanesulfonate; trifluoroacetic acid / dichloromethane / 0.17 h / -40 - -25 °C
4.1: boron trichloride / dichloromethane / 1 h / -20 - -15 °C
5.1: acetone / 0.5 h / 20 °C
6.1: potassium carbonate / water; ethyl acetate
7.1: magnesium chloride; N-ethyl-N,N-diisopropylamine / acetonitrile / 4 h / 20 °C
8.1: hydrogenchloride; water / tetrahydrofuran / 0 - 20 °C
With hydrogenchloride; chloro-trimethyl-silane; trimethylsilyl trifluoromethanesulfonate; water; acetic anhydride; boron trichloride; potassium carbonate; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine; trifluoroacetic acid; magnesium chloride; In tetrahydrofuran; dichloromethane; water; ethyl acetate; acetone; acetonitrile;
|
|
|
Multi-step reaction with 8 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.5 h / 0 °C
2.2: 0.58 h / 0 - 5 °C
3.1: dichloromethane / 0.17 h / -78 °C / Inert atmosphere
3.2: 1 h / -78 °C / Inert atmosphere
4.1: boron trichloride / dichloromethane / 1 h / -78 - -20 °C / Inert atmosphere
5.1: acetone / 0.5 h / 20 °C
6.1: potassium carbonate / water; ethyl acetate
7.1: magnesium chloride; N-ethyl-N,N-diisopropylamine / acetonitrile / 4 h / 20 °C
8.1: hydrogenchloride; water / tetrahydrofuran / 0 - 20 °C
With hydrogenchloride; chloro-trimethyl-silane; water; acetic anhydride; boron trichloride; potassium carbonate; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine; magnesium chloride; In tetrahydrofuran; dichloromethane; water; ethyl acetate; acetone; acetonitrile;
|
|
|
Multi-step reaction with 8 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.5 h / 0 °C
2.2: 0.58 h / 0 - 5 °C
3.1: trimethylsilyl trifluoromethanesulfonate; trifluoroacetic acid / dichloromethane / 0.17 h / -40 - -25 °C
4.1: boron trichloride / dichloromethane / 1 h / -20 - -15 °C
5.1: acetone / 0.5 h / 20 °C
6.1: potassium carbonate / water; ethyl acetate
7.1: magnesium chloride; N-ethyl-N,N-diisopropylamine / acetonitrile / 4 h / 20 °C
8.1: hydrogenchloride; water / tetrahydrofuran / 0 - 20 °C
With hydrogenchloride; chloro-trimethyl-silane; trimethylsilyl trifluoromethanesulfonate; water; acetic anhydride; boron trichloride; potassium carbonate; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine; trifluoroacetic acid; magnesium chloride; In tetrahydrofuran; dichloromethane; water; ethyl acetate; acetone; acetonitrile;
|
|
|
Multi-step reaction with 7 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane / tetrahydrofuran / 0.33 h / 20 °C / Inert atmosphere
2.2: 0.17 h / -78 °C
3.1: dichloromethane / 0.17 h / 0 °C / Inert atmosphere
3.2: 1 h / 0 °C
4.1: boron trichloride / dichloromethane / 1 h / -78 °C / Inert atmosphere
5.1: sulfuric acid / acetone / 0.5 h / 45 °C
6.1: magnesium chloride / acetonitrile / 0.25 h / 20 °C
6.2: 4 h
7.1: water; hydrogenchloride / tetrahydrofuran / 0 - 20 °C
With hydrogenchloride; chloro-trimethyl-silane; sulfuric acid; water; acetic anhydride; boron trichloride; dimethyl sulfoxide; magnesium chloride; In tetrahydrofuran; dichloromethane; acetone; acetonitrile;
|
|
|
Multi-step reaction with 7 steps
1.1: acetic anhydride; dimethyl sulfoxide / 48 h / 20 °C / Inert atmosphere
2.1: chloro-trimethyl-silane; phenylmagnesium chloride / tetrahydrofuran / 0.5 h / 0 - 5 °C / Inert atmosphere
2.2: 0.17 h / -15 - -12 °C
2.3: 1 h / -20 °C
3.1: dichloromethane / 0.17 h / 0 °C / Inert atmosphere
3.2: 1 h / 0 °C
4.1: boron trichloride / dichloromethane / 1 h / -78 °C / Inert atmosphere
5.1: sulfuric acid / acetone / 0.5 h / 45 °C
6.1: magnesium chloride / acetonitrile / 0.25 h / 20 °C
6.2: 4 h
7.1: water; hydrogenchloride / tetrahydrofuran / 0 - 20 °C
With hydrogenchloride; chloro-trimethyl-silane; sulfuric acid; phenylmagnesium chloride; water; acetic anhydride; boron trichloride; dimethyl sulfoxide; magnesium chloride; In tetrahydrofuran; dichloromethane; acetone; acetonitrile;
|
|

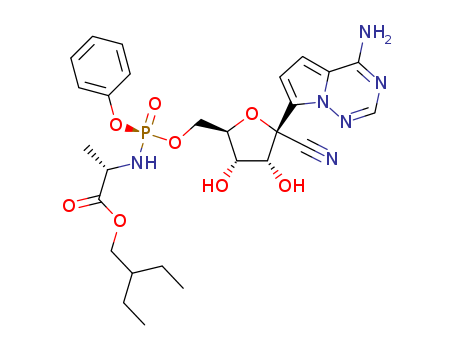

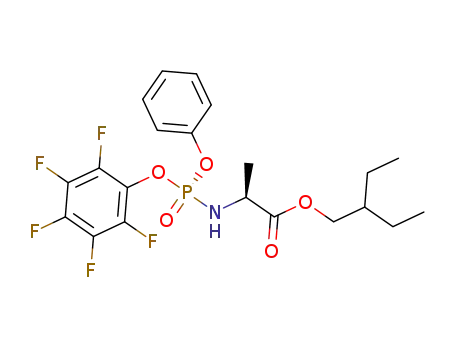
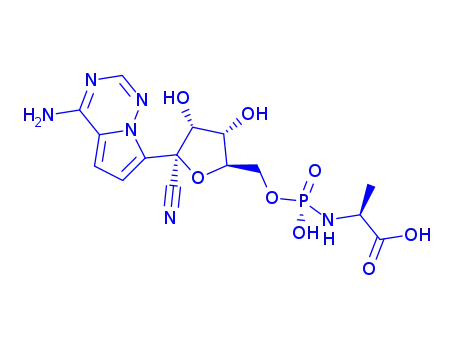
![(2S)-2-ethylbutyl 2-(((S)-(((2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate](/upload/2023/8/2b9eef44-624d-47fd-9e31-368ace856b21.png)

![(3αR,4R,6R,6αR)-4-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-6-(hydroxymethyl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole-4-carbonitrile](/upload/2023/8/f40ffd9d-b8b6-4774-870e-ce182e29ebb4.png)